Open Menu
Out-of-Band Management
AI-Driven Automation and Console Access for Enterprise Networks
Software
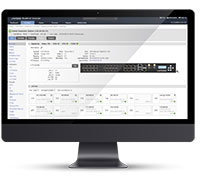
Lantronix Centralized Management Software
Advanced Out-of-Band Management for LM-Series Devices & Connected Network Infrastructure
Services
Services
SOMs & Dev Kits
Embedded Controllers and Systems